Click for more images: Optical System
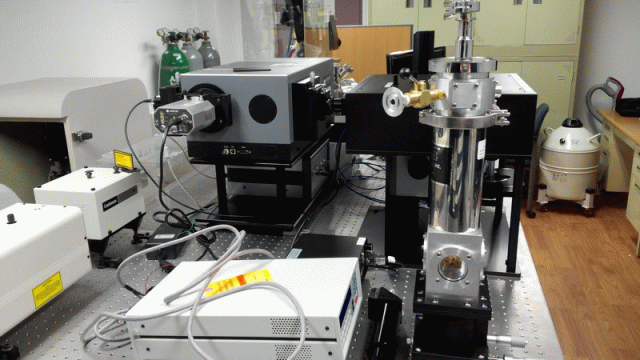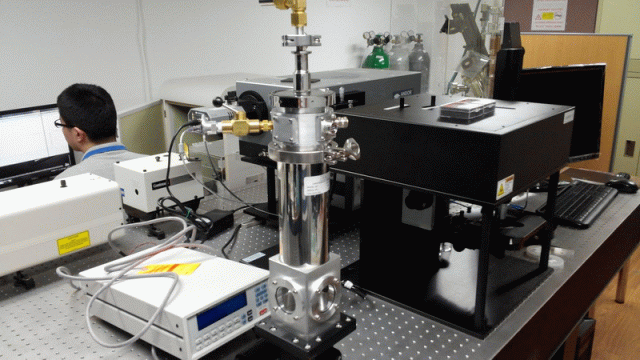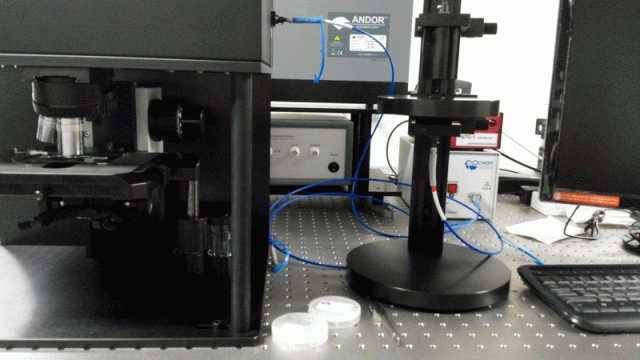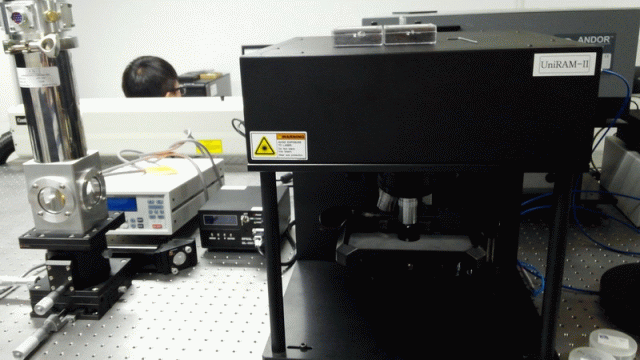
< PL/Raman/ATR combined system: Prof. Jihoon Lee’s group
at Kwangwoon Univ. >
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation).
It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photons that excite electrons to a higher energy level in an atom), hence the prefix photo-.
Following excitation various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for Phosphorescence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.
Observation of photoluminescence at a certain energy can be viewed as an indication that an electron populated an excited state associated with this transition energy.
(Source: Wikipedia)
Raman spectroscopy (named after Sir C. V. Raman) is a spectroscopic technique used to observe vibrational, rotational, and other low-frequency modes in a system.
Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used.
The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar, complementary, information.
(Source: Wikipedia)
ATR (Absorption Transmittance Reflectance)
In physics, absorption of electromagnetic radiation is the way in which the energy of a photon is taken up by matter, typically the electrons of an atom.
In optics and spectroscopy, transmittance is the fraction of incident light (electromagnetic radiation) at a specified wavelength that passes through a sample.
Reflectivity or reflectance is the fraction of incident electromagnetic power that is reflected at an interface, in contrast to the reflection coefficient, which is the ratio of the reflected to incident electric field.
(Source: Wikipedia)
Click for more images: Optical System
